Kinetic Molecular Theory of Heat
The Particle Theory of matter explains that all matter is made up of particles and that all particles are always in constant motions.
The diagrams below illustrate the differences in the types of energies that the three states of matter display.
- Solids - Vibration Energy
- Liquids -Vibration & Translational Energies
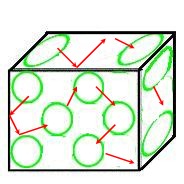
- Gases - Vibration, Rotational, Translational Energies
Particles in motion have kinetic energy.
This kinetic energy is an indirect measure of the amout of heat (Thermal Energy) content that an object has.
This heat content depends on:
- the motion and speed of the particles (temperature),
- the amount of particles (mass),
- and the type of material (heat constant).


