The Nature of Electricity
Anything around us that has mass and occupies volume is known as matter.
Matter is made up of tiny particles called atoms. Atoms join together to form molecules.
Atoms are made up of three fundamental particles: neutrons, protons, and electrons.
According to Rutherford’s model of the atom, there is a dense, positively charged nucleus, in which all the mass is concentrated, surrounded by a region of negatively charged electrons.
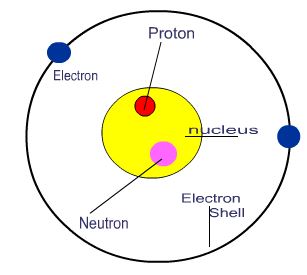
The diagram above is a representation of the Bohr-Rutherford Model of the Atom
Te electrons are in the outer shell while the protons and neutrons are ocated in the nuckeus
Electrons are negatively charged particles that move in circular orbits around the nucleus of an atom.
Electrons are the particles of the atom that are responsible for bonding (the electro-chemical process of combining two ore more atoms into molecules). Electricity can be explained by investigating the properties and behaviour of electrons.
Electrons are the particles of the atom that are responsible for bonding (the electro-chemical process of combining two ore more atoms into molecules). Electricity can be explained by investigating the properties and behaviour of electrons.
Electric Charges
Neutral object have no charge.
This means that the number of positive charges on the object equals the number of negative charges. We can charge an object by rubbing it with another object.
Charging is a physical change; a process that will either take away negative charges from an object or add negative charges to it.
An object that has more negative charges than positive charges is called a NEGATIVELY CHARGED object.
An object that has LESS negative charges than positive charges is called a POSITIVELY CHARGED object.
An object that has LESS negative charges than positive charges is called a POSITIVELY CHARGED object.
-
A loss of electrons = a positive charge
-
A gain of electrons = a negative charge
Neutral Objects can be attracted by either positively or negatively charged objects.


