Atomic Theories
The idea of an atom -- the smallest particle of matter -- has intrigued mankind since the beginning of civilization. Throughout the centuries the "view" of the atom has changed. New ideas, and new technologies have influenced the model of the atom. This view of the atom is still a Theory and therefore it is still subject to change. The modern model of the atom is called the Quantum Model and you will study this model in future grades (Grade 11 and 12 Chemistry & Physics).
Summarized below are the various atomic models that have been developed during the course of history.

Democritus, c.300 BC
Atom the indivisible particle: Atomos (in ancient Greek) means "that which cannot be further broken down into smaller pieces"
- Talks about the atom as the smallest particle of matter.
- Defines the atom as an indivisible particle
- Explains certain natural occurrences such as the existence of elements
Shortcomings:
- Does not give a scientific view of the atom only a conceptual definition
- Does not talk about subatomic particles (Electrons, Protons, Neutrons)
Dalton, c.1800
The solid sphere model: Atoms are seen as solid, indestructible spheres (like billiard balls)
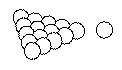
- Explains a lot of chemical properties such as how atoms combine to form molecules
- Explains chemical change better than the Particle Theory
- Confirms the basic Laws of Chemistry: Conservation of Mass & definite Proportions
Shortcomings:
- Does not include the existence of the nucleus
- Does not explain the existence of ions or isotopes
- Does not talk about subatomic particles: (Electrons, Protons, Neutrons)

J.J. Thomson, c.1850
The raisin bun Model or the chocolate chip cookie model: Atoms are solid spheres made-up of a solid positive mass (or core) with tiny negative particles embedded in the positive core.

- Infers on the existence of electrons and protons
- Introduces the concept of the nucleus
- Infers on the relative nuclear density and atom mass of different atoms
Shortcomings:
- Does not explain the existence of electrons outside the nucleus does not explain the role of electrons in bonding
- Does not talk about neutrons therefore can't explain radioactivity and the existence of isotopes

Rutherford, c. 1905
The Planetary Model: The Famous Gold Leaf Experiment proved that the nucleus is positive and the electrons are outside the nucleus.
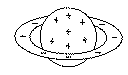
- First real modern view of the atom
- Explains why the electron spins around the nucleus - (Bohr's Contribution)
- Proposes that the atom is really mostly empty space
Shortcomings:
- Does not place electrons in definite energy levels around the nucleus
- Doesn't include neutrons in the nucleus
- Does Not relate the valence electrons atomic charge

(Neils Bohr), Bohr- Rutherford c. 1920
Electrons in Definite energy Levels around the nucleus: Used atomic spectra to prove that electrons are placed in definite orbitals (called shells) around the nucleus.
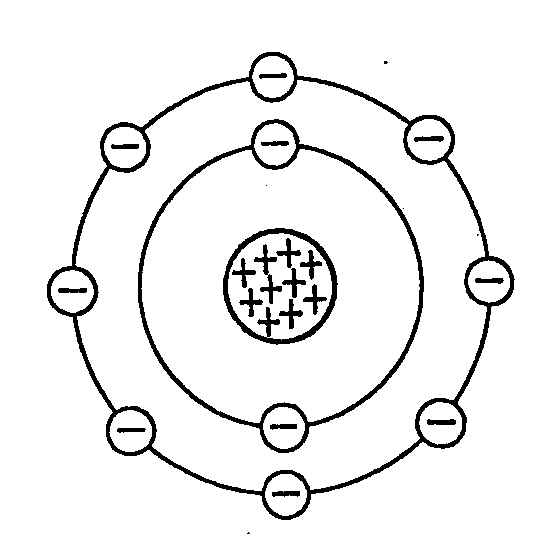
- Explains the role of valence electrons in bonding
- Relegates the number of valence electrons to the Periods of a periodic table
- Fully explains ionic and covalent bonding
-
Places electrons in definite energy levels
- 2 e- in the first
- 8 e- in the second
- 8 e- in the third
Shortcomings:
- It does not explain the shapes of molecules or other abnormalities that result form unevenly shared pairs of electrons (such as the abnormal behaviour of water, the difference in Carbon-Carbon Bonds between diamond and graphite etc..)
Modern Theory
Many scientists contributed to this theory including:
- Schroedinger
- Einstein
- Luis De Broglie
- Max Planck
- Frank Hertz
- Maxwell
- Fermi
This model of the atom is commonly known as the Quantum Mechanical Model or Electron Cloud Model. The analogy here is that of a "beehive" where the bees are the electrons moving around the nucleus in a "cloud" of energy levels - Advanced Theories will explain bonding and other facts about the behaviour of atoms and their chemical and physical properties in forming new compounds.
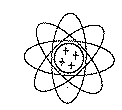
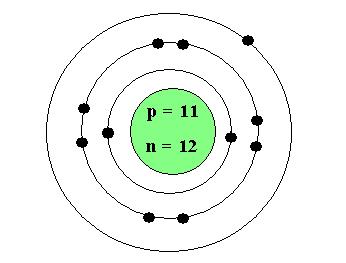
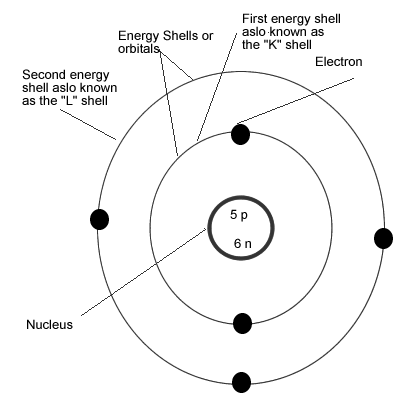
The Bohr-Rutherford Model (Helium Atom)
Other important facts about the particles of an atom:
| Subatomic Particle | Symbol | Charge | Relative Mass* | Location |
| Proton | p+ | positive | 2000 | nucleus |
| Electron | e- | negative | 1 | orbits around nucleus |
| Neutron | n0 | neutral (zero) | 2000 | nucleus |
*Relative mass means that is the electron has a mass of 1 unit, the proton and neutron will have a mass 2000 times that of the electron.
The mass number (also known as atomic mass or atomic weight) and the atomic number from the Periodic Table are very important numbers because they tell us how many subatomic particles are contained in a given atom.
The atomic number tells us the number of electrons and the number of protons., i.e. Atomic Number = Number of electrons = Number of Protons. The atomic mass tells the total number of particles in the nucleus, i.e. Atomic Mass = # of protons + number of neutrons.
For example: The square where the element Boron is located on the Periodic Table looks like this
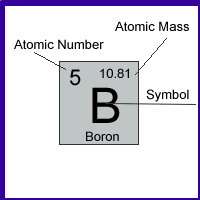
From this we can obtain the following information:
| Element | Symbol | Atomic Number | Atomic Mass | # of protons | # of electrons | # of neutrons |
| Boron | B | 5 | 11 | 5 | 5 | 11 - 5 = 6 |
Recall that the Bohr-Rutherford Model places the electrons around the nucleus in definite orbitals or energy shells as summarized by the table below.
| Energy Shell |
Name of Shell or Symbol |
Maximum Number of Electrons it can contain |
| 1 | K | 2 |
| 2 | L | 8 |
| 3 | M | 8 |
| 4 | N | 18 |
Now we use this and the information from the Periodic Table to draw a Bohr-Rutherford diagram for the Boron Atom as illustrated below:
- To draw Bohr-Rutherford diagram for Boron we place the first 2 electrons in first shell.
- The first shell can only hold a maximum of 2 electrons so we start filling the second shell
- The Boron atom has 5 electrons therefore we have another 3 electrons to place
- We place these electrons in the Second shell and we space them apart from one another
If there are more than four electrons in the second shell (as in the case of the Fluorine atom), we pair the electron up. This pairing of electrons is explained by more advanced theories which propose that to counteract the repulsive forces between the electrons' negative charges, one elctron spins in the opposite direction of the second electron.
Activity:
Draw a Bohr-Rutherford diagram for the element Sodium.
Solution:
Using the periodic table we obtain the following information about the sodium atom., Na:
| Atomic Number | Atomic Mass |
# of protons (and electrons) |
# of neutrons |
| 11 | 23 | 11 | 12 |
Completed diagram: