An element is the smallest portion of matter of a specific substance.
Elements are the building blocks of all that is matter.
An element cannot be broken down any further by chemical change. For example, the element Oxygen is the smallest portion of oxygen gas.
It is now possibe to make artificial elements through nuclear reactions.
It is not possible to create elements using simple chemical reactons.
All elements and their properties are listed on the periodic Table of the Elements.
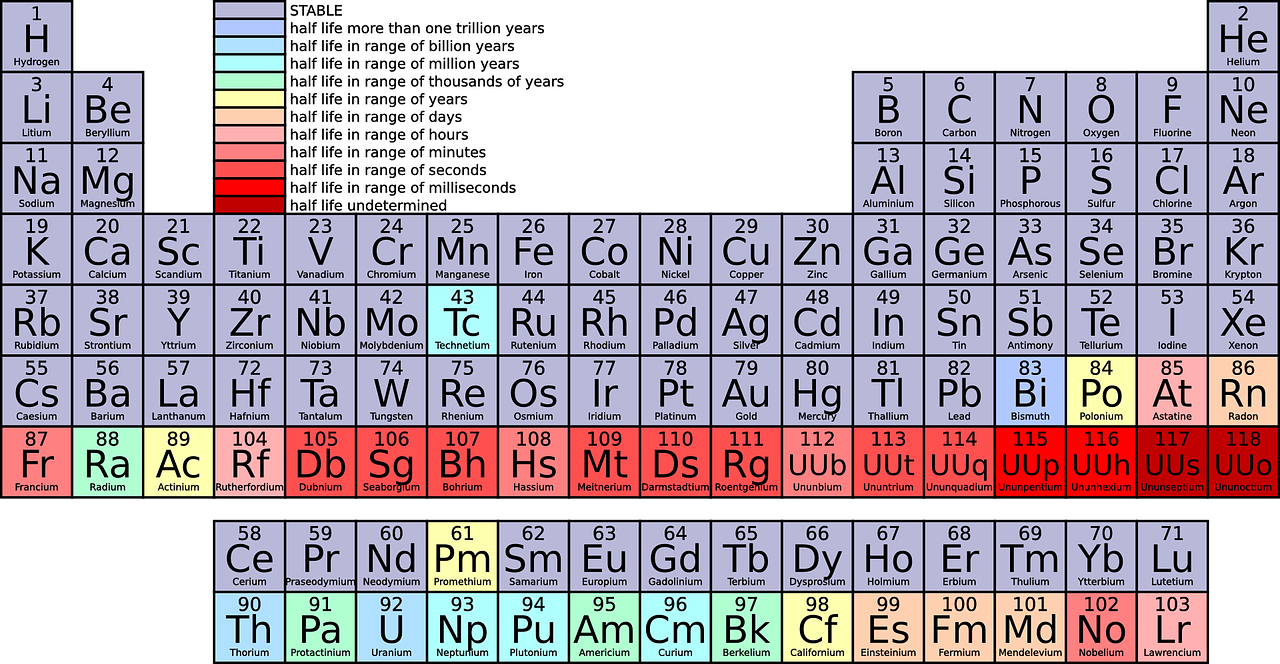
As of now there are only 118 elements known to scientists, including those that were artificially made in a lab.
A compound is a substance made up of two or more elements.
Chemical changes can break down compounds into simpler compounds or elements or they can form compounds by combining different elements.
A fancy word for combining substances to make new ones during chemical reactions is synthesis.
Physical changes cannot break down compounds.
There are millions of different compounds known to us, but there are many more that are yet to be discovered by scientists.
Compounds either occur naturally or have been manufactured by man through chemical reactions (chemical changes).
Here are some examples of everyday compounds you are familiar with:

Water is made up of 2 parts of the element hydrogen + one part of the element Oxygen.

Table Salt (known in Chemistry as Sodium Chloride) is made up of 1 part of the element Sodium + 1 part of the element Chlorine.


